


Written by Rick

How to pump Sulfuric Acid
The selection of the pump for sulfuric acid (H2SO4) must always take into consideration the acid concentration with its specific gravity, temperature, and the working conditions of the pump. In general, it is difficult to find material which can resistance over all concentration range and temperature of sulfuric acid. There are only few special, expensive metal alloys, which can handle sulfuric acid in the concentration range of 0 – 99%. Commonly, different pump materials are used for specific acid concentrations.
In general, plastic materials are recommended for low concentrated sulfuric acid. However, when selecting plastic pumps their temperature limitations should be taken into account.
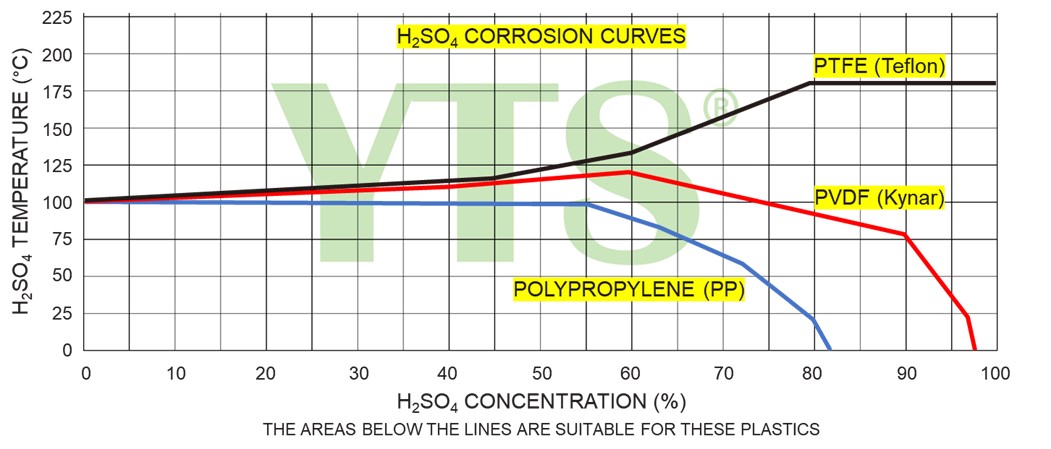
Temperature constructional limits of plastic pumps must be taken into consideration to preserve enough mechanical stability of pump parts.
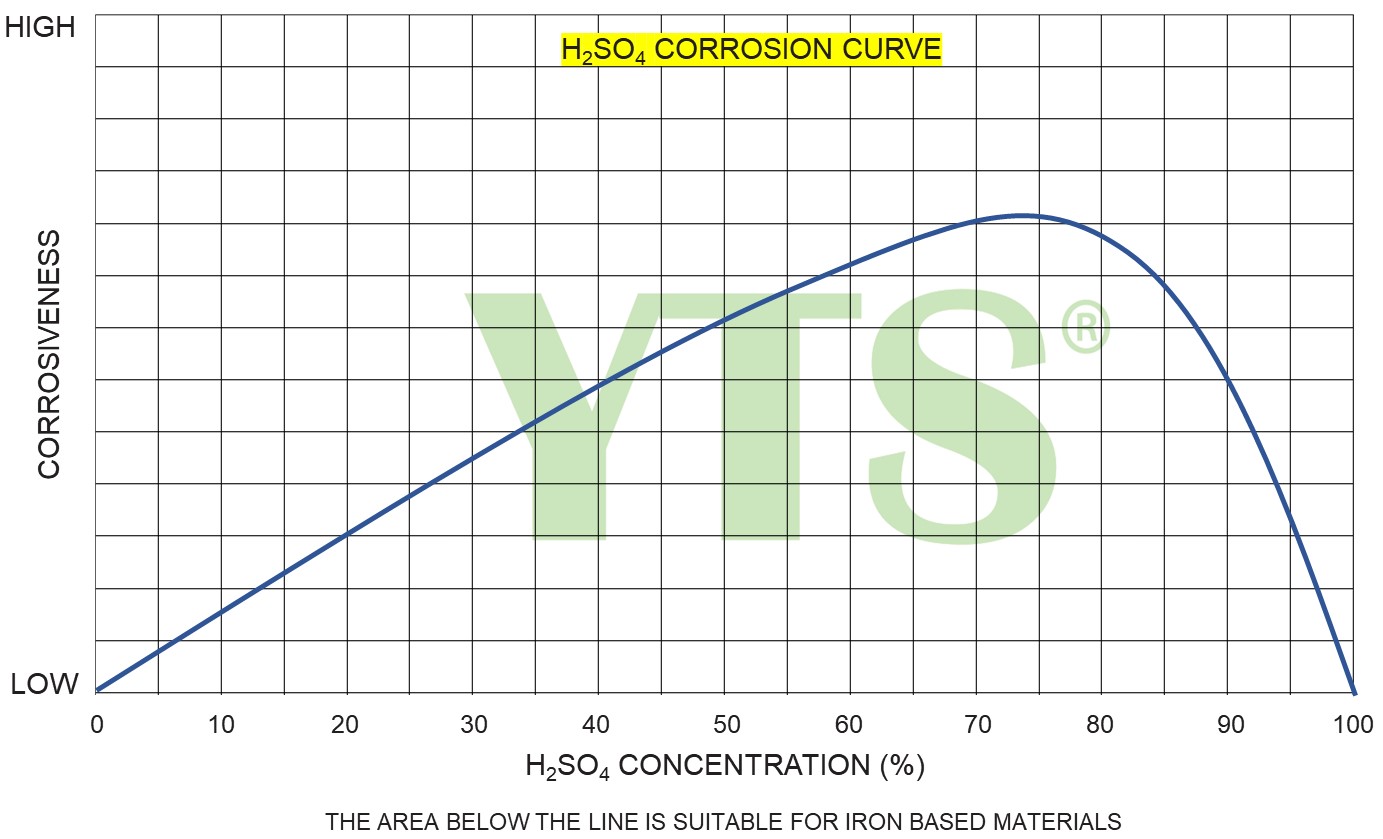
Stainless Steel SS316 (DIN 1.4408) typically used for pumping sulfuric acid has a good resistance in the concentration range 80 – 99%. SS316 can be also used for low concentrated H2SO4 in the concentration range up to 25%. The high concentration of sulfuric acid (95 – 99%) may be pumped using cast iron materials.
One of the most versatile alloys for use with sulfuric acid is corrosion resistant Hastelloy, which has excellent resistance to uniform corrosion in oxidizing or reducing environments, excellent resistance to stress corrosion cracking and superior resistance to localized corrosion. YTS Hastelloy pumps possesses outstanding resistance to sulfuric acid across whole range of concentrations.
When handling sulfuring acid, the special attention should be paid to piping system, particularly to the weak points in any piping system – connections, in-line instruments, valves, and equipment. When the fluid carried is hot concentrated H2SO4 any leaks at these connections becomes a major safety concern.
In sulfuric acid piping system, all connections should be flanged connections. Screwed connections are not recommended under any circumstance. To protect flange connections especially in the systems, which operates under high pressure flange guards are used. The flange guard works as a shield, enclosing the flange.
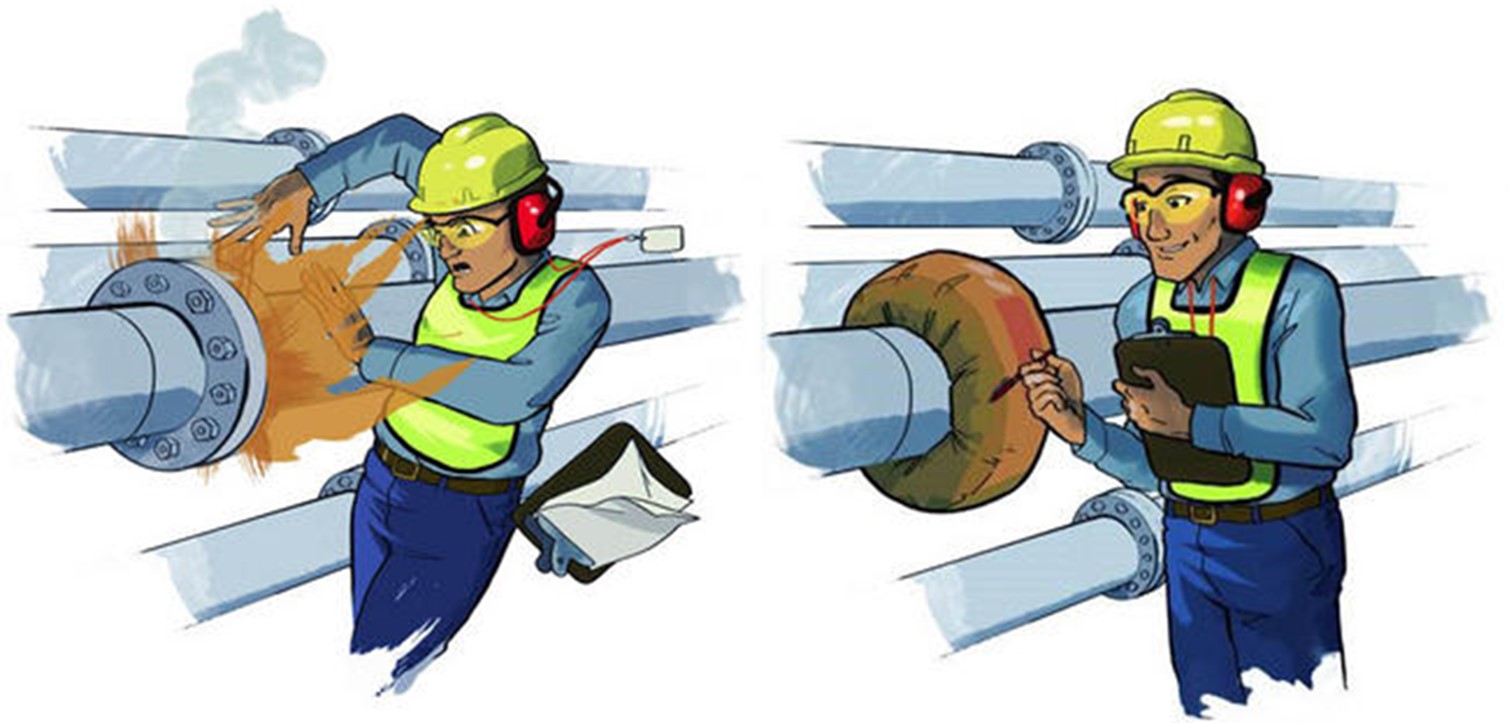
In case of the leakage the flange guard prevents acid spraying out into the operating area. The spray is contained, and is converted into droplets which are less hazardous.
Working conditions of the pump – type of pump installation – is another factor, which has to be investigated at length when selecting pump for sulfuric acid.
H2SO4 specific gravity (density) varies from 1 to 1,84 g/cm³ depending on acid concentration, the temperature and the pressure. This parameter is of great importance for calculating pump operating parameters in a specific installation. Particular attention is required for AODD pumps installations with positive inlet pressure.
Rubber diaphragms (Neoprene, EPDM, Viton) and thermoplastic diaphragms (Santoprene, PTFE) which may be use with certain concentration of the acid, all have limits of permissible positive inlet pressure (Net Positive Suction Head – NPSH). Special care is required in those cases in which PTFE diaphragms are used.
Sulfuric acid – “king of chemicals” is the most commonly used chemical in the world. It is used as a raw material or processing agent in almost all industries. For chemical industry sulfuric acid is one of the most important substance that is used to make hundreds of others chemical compounds (like for example hydrochloric acid, nitric acid, phosphoric acid) widely utilized in many industries. Several examples:
- Almost 50% of the worldwide sulfuric acid is used to produce phosphate fertilizers. Phosphate fertilizers are crucial for agriculture for handling sulfur deficiency.
- Sulfuric acid is widely used in the mining/metallurgy industry for dissolution of ores, to recover different types of metals (copper, gold, silver, zinc, etc.).
- In seawater desalination plants sulfuric acid is used for removing salts and minerals from saline water, pH adjustment of sea water.
- In paint industry sulfuric acid is used as a reagent in the synthesis of pigments for paint.
- During refining of crude oil sulfuric acid is utilized as a catalyst.
- In metal processing sulfuric acid is used in pickling process (process of removing impurities, rust or scale from the surface of the metal).
- In lead-acid type batteries which are used in cars and truck sulfuric acid act as an electrolyte allowing the flow of electrons between the plates in the battery. H2SO4 (also called Battery Acid) is used in a dilute form with concentration between 28% to 32%.
- In agriculture, in mass potatoes production sulfuric acid is utilize to spray fields of potatoes before harvesting to dry out green tops. This helps to dry out the stem and prevents them from becoming tangled in the harvesting equipment.
Sulfuric acid is non-flammable in its natural state, however extremely dangerous is formation of hydrogen, that occurs when sulfuric acid reacts with metals (iron, aluminium, zinc, manganese, magnesium, nickel). The result of this reaction is formation of salt (sulfate) and hydrogen.
Hydrogen can form a flammable mixture with air over a wide range of concentrations (from 4 to 75%). The formed hydrogen will find, in every plant, stagnant areas where gas can accumulate and form an explosive hydrogen-air mixture. And very low energy is needed to ignite hydrogen-air mixtures. A spark with such low energy that it is invisible in a dark room can ignite hydrogen-air mixture. Common sources of ignition are sparks from electrical equipment, and sparks caused by the discharge of a small accumulation of static electrical charges.
Sulfuric acid is very toxic, corrosive, fatal if inhaled, can form very hazardous decomposition products, highly reactive, incompatible with many common chemicals, reacts violently with water (water/humidity ingress to the acid must be prevented), causes severe skin burns and eye damage, aggressiveness rises exponentially with the temperature. Therefore, handling of concentrated and dilute solutions must take place with special safety measures. Vital for the safety is a leak free operation of pump, and leak free piping system.
We’ve got something exciting to tell you!
HELLO SWEDISH! We’re excited to announce that we’ve just launched Swedish language support on our website! This new addition to our language family has been

Indoor Air Quality
Indoor Air Quality (IAQ) – air quality within and around buildings and structures plays is of utmost importance not only for employees well-being and ability

10 Factors to Consider When Selecting Diaphragm
Diaphragms are one of the most important elements of Air Operated Double Diaphragm Pump. They separate the wetted side (fluid side) of the pump from

28 Reasons Why Use Air Diaphragm Pump
Air Diaphragm Pumps are so much versatile in design, materials, performances and functions, that they can handle most types of fluids. They are used for

Hastelloy Air Diaphragm Pumps
The primary function of Hastelloy C-22 (also known as a “superalloy” or “high-performance alloy”) is a long-lasting survival in severely corrosive, or erosion prone environments,

Too Small Size of Air Supply Line to the Air Diaphragm Pump
It is not uncommon to use too small air supply line when installing an Air Operated Diaphragm pump. Overlooking restrictions installed along the line, which
