


Written by Rick

How to pump Sodium Hydroxide
Sodium hydroxide NaOH (common names – caustic soda, soda lye, white caustic, sodium hydrate) is one of the most commonly used industrial chemicals. It is very often used for pH control (to raise it) as it is very alkaline. The most used concentration of NaOH is 50%.
The major consideration during pumping sodium hydroxide (caustic soda) should be paid to:
1. Chemical resistance of the pump
Caustic soda is highly corrosive base, and depending on the concentration of the solution different materials should be used. In concentrations up to 70% at the ambient temperature sodium hydroxide can be handled either by stainless steel, cast iron or plastic pumps.
Note: NaOH solutions with concentrations above 50% are not common. NaOH concentrations above 50% can’t be prepared at ambient temperature (around 20°C). For example, 60% solution has to be heated to at least 60°C.
Pure Polypropylene has “A” grade against all concentration strengths of sodium hydroxide at temperatures ranging from 20°C to 100°C. Polypropylene is more resistant to NaOH corrosive effects, than HDPE and XLPE. Fiber Glass Polypropylene has limited resistance to sodium hydroxide as caustic soda corrosively attacks the glass fibers that comprise pump body. This may cause cracks formation in the pump housing and possible fluid leakage. Pumps of smaller sizes are much less exposed to this occurrence, than 2″ or 3″ pumps.
Stainless steel at 50% (pH around 14) concentration begin to experience elevated stress corrosion cracking at temperatures in excess of 49°C.

Aluminium can’t be used – sodium hydroxide corrodes aluminum and during this process generates hydrogen, which has the potential to behave as an explosive gas.
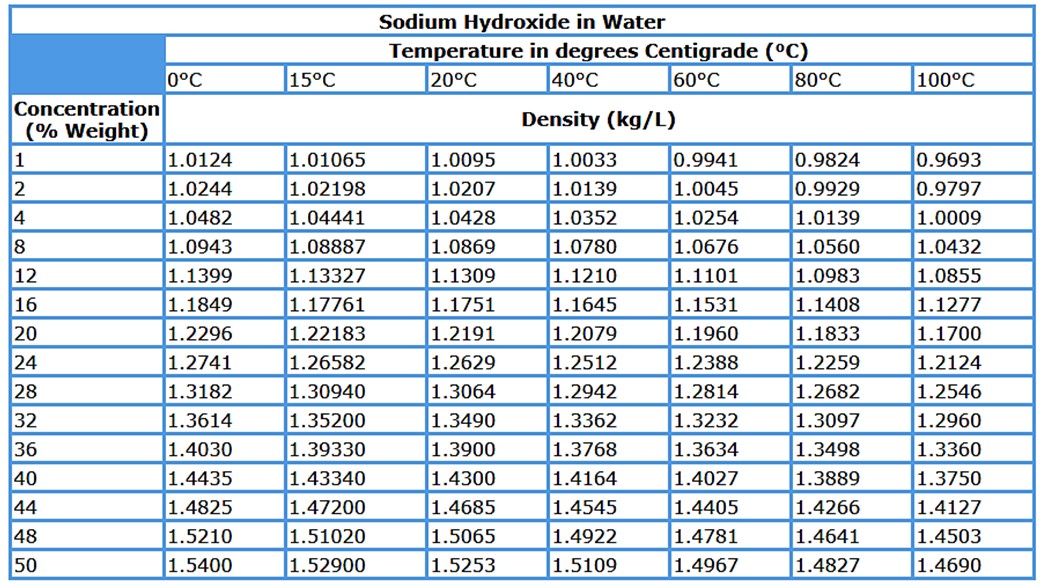
2. Density
Attention should be paid to density of sodium hydroxide. It varies greatly depending on concentration and temperature.
3. Freezing point
Special attention should be paid to the freezing point of sodium hydroxide. NaOH freezing points vary markedly at various concentrations. As the temperature is near the freezing point, NaOH can begin to crystallize. Buildup solids within the pump will affects pump performance and can damage diaphragms. Buildup solids in the piping system will restrict fluid flow. Temperature of freezing points for concentrations:
5% -4°C
10% -10°C
30% 1°C
50% 12°C
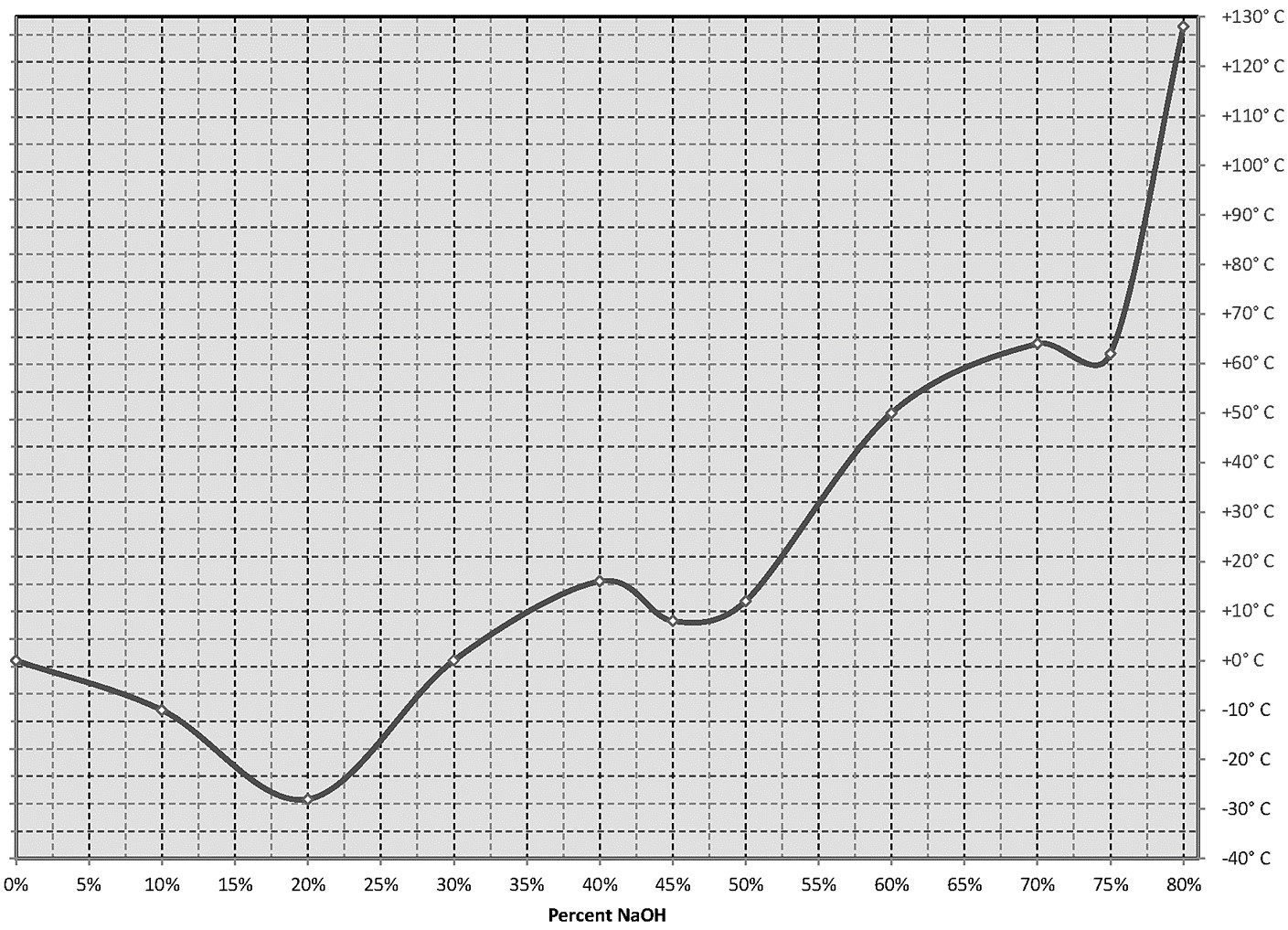
Due to NaOH freezing point characteristic indoor location of the pump is recommended to secure temperature stability. For outdoor operations, where temperatures beneath 20°C are expected, more dilute solutions of NaOH are used (their freezing points are lower). If freezing is the case, the use of heated equipment will solve the problem – suitable heated pipes, heat jacket on the pump, sometimes even proper insulation is enough.
4. Viscosity
Sodium hydroxide viscosity varies with concentration. At 20°C, a 50% NaOH solution will have a viscosity rating around 79cP (it will be like an olive oil – 84cP). This is important characteristic which should be considered. Especially when selecting small size pumps for dosing applications.
Caustic Soda is highly corrosive, have a decomposing effect on proteins. Even a dilute solution can affect the tissue of the skin, which may cause dermatitis or chronic eczema. If the concentration of NaOH solution is high, the affected tissue rapidly decomposes. In particular, the eyes are very vulnerable, since eye tissue is rapidly affected causing a lowering or loss of vision. NaOH may cause an exothermic reaction in contact with other fluids, particularly with water. That is why, it is so important to ensure trouble-free pump operation without any spillages of the fluid.
Sodium hydroxide is so versatile alkali, that is used by most industries. The largest users of sodium hydroxide are the pulp and paper, detergent and chemical industries. Several examples of application:
• The production of paper uses a significant proportion of the world’s caustic soda. The solution is used to separate lignin from cellulose fibers in the production of pulp. The solution is used again as part of the bleaching process to obtain the brightness of the paper product and in deinking.
• Caustic soda is used for pH correction as part of chemical production and water and sewage treatment.
• NaOH has a very wide range of uses in food, diary and beverage industries. It is used in CIP processes. For the cleaning and peeling of fruits and vegetables as well as a brine solution for the softening of olives. For chocolate and cocoa processing and many others.
• In heavy industry caustic soda is used for processing metals and glass products. And in thermal power stations NaOH is used to react with the carbon dioxide in flue gas desulfurization process.
• In household caustic soda is used in chemicals for drain unblocking (food, fats and hairs are quickly dissolved), in cleaning chemicals (fats and grease are dissolved), in cosmetic products – hair softening liquids, soaps.
We’ve got something exciting to tell you!
HELLO SWEDISH! We’re excited to announce that we’ve just launched Swedish language support on our website! This new addition to our language family has been

Indoor Air Quality
Indoor Air Quality (IAQ) – air quality within and around buildings and structures plays is of utmost importance not only for employees well-being and ability

10 Factors to Consider When Selecting Diaphragm
Diaphragms are one of the most important elements of Air Operated Double Diaphragm Pump. They separate the wetted side (fluid side) of the pump from

28 Reasons Why Use Air Diaphragm Pump
Air Diaphragm Pumps are so much versatile in design, materials, performances and functions, that they can handle most types of fluids. They are used for

Hastelloy Air Diaphragm Pumps
The primary function of Hastelloy C-22 (also known as a “superalloy” or “high-performance alloy”) is a long-lasting survival in severely corrosive, or erosion prone environments,

Too Small Size of Air Supply Line to the Air Diaphragm Pump
It is not uncommon to use too small air supply line when installing an Air Operated Diaphragm pump. Overlooking restrictions installed along the line, which
